What can you learn from this article?
An experimental cohort study design for policy evaluation, where countries made the move from a two-dose to single-dose schedule for national HPV vaccination programmes since 2023.
Introduction
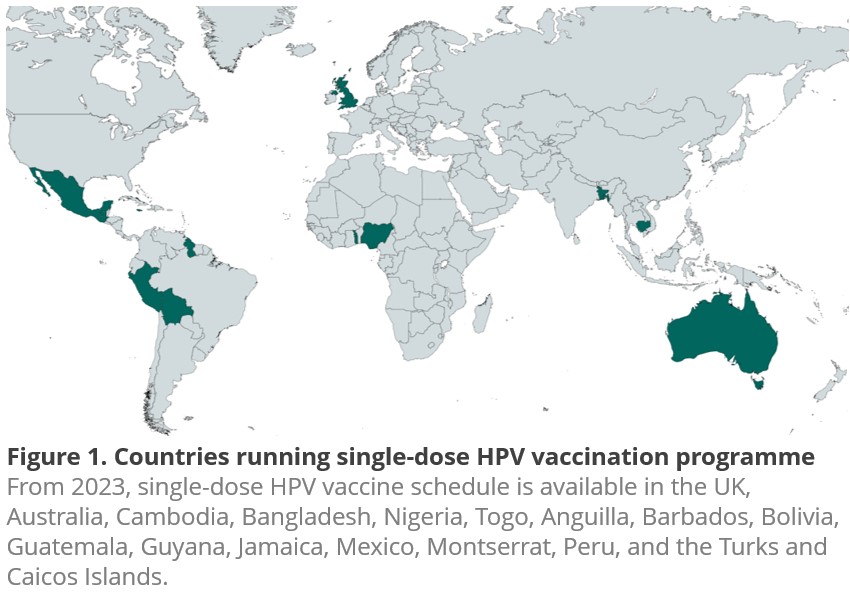
Since the first licensing of the Human Papillomavirus (HPV) vaccine in 2006, evidence has been emerging showing that single-dose schedules provide comparable efficacy to the conditional regimens, i.e. two or three doses. In 2022, a review of World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) concluded that a single-dose HPV vaccine delivers solid protection against HPV. Consequently, there have been 16 countries that adopt SAGE’s recommendation of single-dose schedule in their national immunization programmes in 2023, including the UK [Figure 1].
This study aims to assess the non-inferiority of the real-world impacts in single-dose HPV vaccination programmes with regard of herd immunity and paradigm shift.
Method
This research is designed as a prospective and closed cohort study with a 20-year follow-up.
Source Population
- Age: adolescent females aged 12 to 13 in 2023-24 in 16 study countries, i.e. age eligible to their national HPV vaccine program.
- Inclusion: get at least one dose of HPV vaccine and undergo screening in the follow-up period.
- Exclusion: those who migrate out of the study countries.
Exposure
- The final vaccination status, categorized into two and three doses of HPV vaccines, is considered as two distinct exposures.
- Single-dose HPV vaccination serves as the reference group (non-exposure).
Outcome
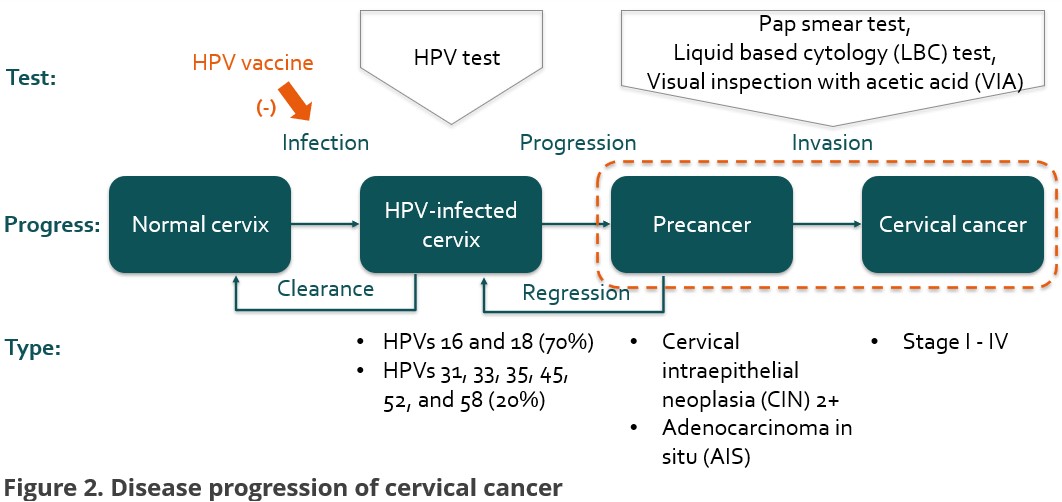
Regarding the disease progression [Figure 2], in the first decade, this study defines precancer as the preliminary outcome, including Cervical intraepithelial neoplasia (CIN) 2+ and Adenocarcinoma in situ (AIS).
Cervical cancer is investigated as the secondary outcome over the last decade. Three kinds of methods are used for screening testing, including pap smear test, liquid based cytology (LBC) test, and visual inspection with acetic acid (VIA).
Data Collection
Follow-up continues until the diagnosis of cervical cancer (or precancer), death, or the end of 2043. The rationale for 20-year follow-up is the highest incidence rates being in the 30 to 34 age group in the UK, that will be the end of their follow-up.
Data integration is streamlined through annual data linkage across five sources from each country, including population immunization registry, cervical screening registry, death registry, national migration record, and regional socio-economic status.
Statistical Analysis
The demographic characteristics are depicted through the mean (± standard deviation) and counts (percentage).
This study uses Cox proportional-hazard regression, adjusted for sexual orientation and socio-economic index, with age as the time axis to estimate hazard ratios (with 95% Confidence Intervals, CIs) of cervical cancer (or precancer) stratified by country according to their vaccine doses.
Non-inferiority is defined when the difference in hazard ratios is less than a 5% deviation from 1.
RESULT (HYPOTHETICAL)
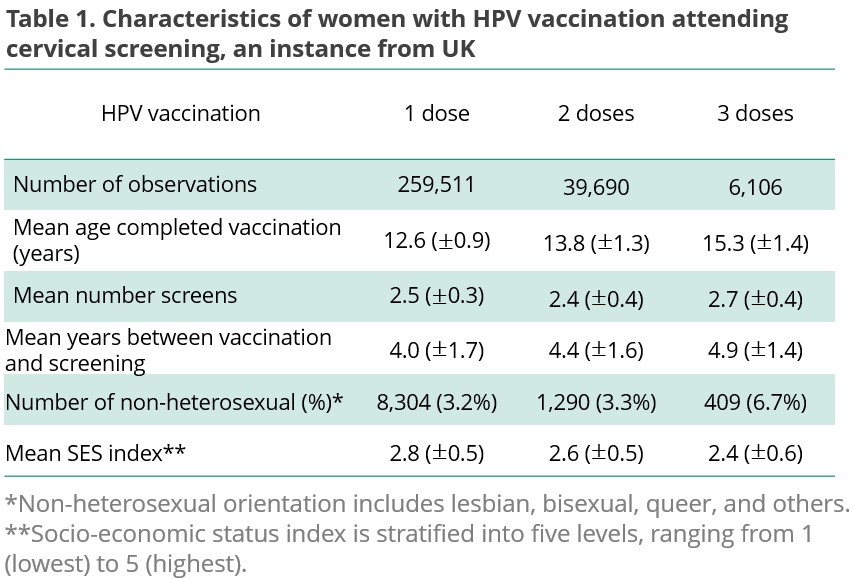
Take UK for instance, the cohort include 305,307 vaccinated females. Of these, 259,511 (85%) received single dose, 39,690 (13%) received two doese, and 6,106 (2%) received three doses. Their mean age completed vaccination is 12.6, 13.8, and 15.3, respectively.
Overall, the average number of screens is more than 2. The years between complete vaccination and screening, sexual orentation, and socio-economic index are detailed in Table 1.
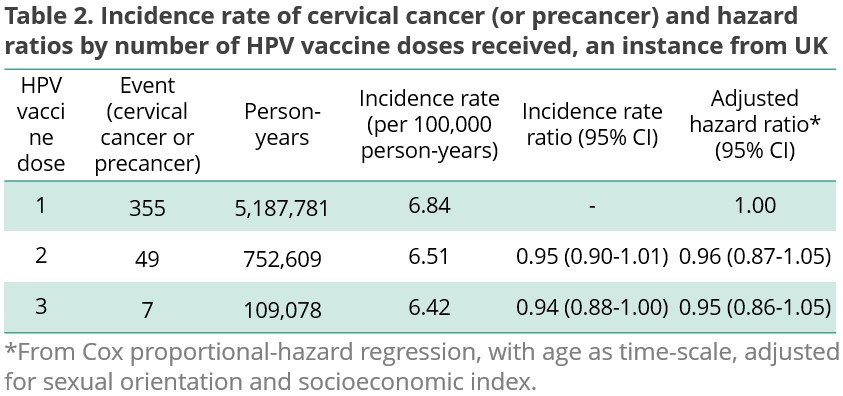
The difference in the incidence rate ratio of three subgroups is negligible (1 dose 6.84, 2 doses 6.51, and 3 doses 6.42, per 100,000 person-years). The adjusted hazard ratio was not significantly lower for 2+ dose groups compared to single-dose women (2 doses 0.96 (95% CI 0.87-1.05) and 3 doses 0.95 (0.86-1.05)), indicating the non-inferiority of single-dose schedule [Table 2].
Of 16 study countries, 14 single-dose HPV vaccination programmes achieve non-inferiority. (The findings from countries outside the UK are omitted.)
DISSCUSION
Strengths
This research exhibits four strengths. First, the substantial sample size enhances the generalizability of findings. Second, Geographical comparisons are attainable thanks to multi-country data sources.
Third, data are reliably obtained, as they are routinely updated in country’s immunization and screening programs. Last but not least, the final disease outcome, i.e. cervical cancer, is used as the study measurement, surpassing previous studies reliant on short-term outcomes, such as HPV persistent infection.
Limitations and bias
However, this research is constrained by its assumptions of not stratifying results according to vaccine brands or screening methods, the later may lead to misclassification due to diverse sensitivity and specificity of the testing.
Unregistered or incorrect record of doses may also cause non-differential misclassification of the exposure. Such information bias is towards the null.
With regard to selection bias, the willingness of screening may be influenced by the final vaccination status. Notably, the exclusion of females who have not been vaccinated in the defined source population is essential to prevent such selection bias.
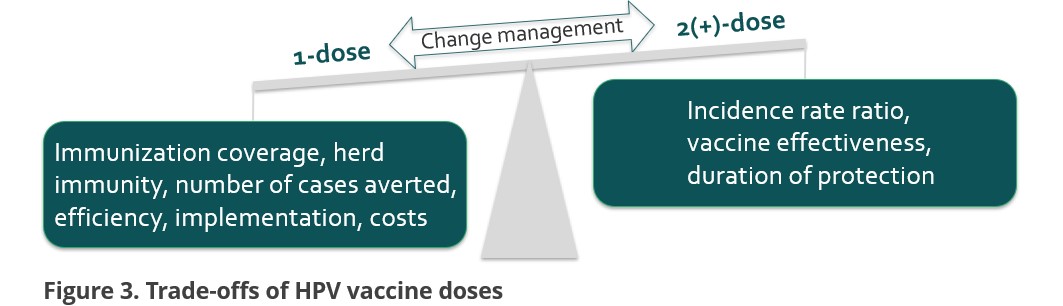
Trade-offs
When it comes to inferiority, this study’s emphasis lies in the trade-offs aspect. On the side of single dose, higher immunization coverage can be obtained, which is conducive to herd immunity. Besides, the number of cases averted will be more appealing to policy makers from a prospective of public health. Not to mention the benefit of efficient implementation and reduced costs.
On the side of 2+ doses, the relative risks exhibit a somewhat lower magnitude. With regard of the waning immunogenicity, some modelling studies suggest that some of the single dose girls need a catch-up dose to obtain sufficient immunity in population.
CONCLUSION (HYPOTHETICAL)
Single-dose HPV vaccination demonstrates comparable efficacy compared to 2+ doses in 14 out of the 16 study countries’ programmes, suggesting that a shift to single-dose schedule is suitable for cervical cancer prevention.
This article has been adapted from the poster, originally created as part of the assessment for the epidemiology module at LSHTM. I express sincere gratitude to Professor Neil Pearce for his exceptional teaching.
